Los Angeles, October 15 (RHC) A New research published Wednesday in The Lancet Journal provides the first evidence of the medium- to long-term safety and tolerability of transplanting human embryonic stem cells (hESCs) in humans.
Steven D Schwartz and along with his colleagues of the retina division at the Jules Stein Eye Institute at the University of California, Los Angeles, reported the outcomes of hESCs for the treatment of macular degeneration and Stargardt’s macular dystrophy as the leading causes of adult and juvenile blindness in the developed world.
Since they were first derived more than three decades ago, embryonic stem cells have been proposed as a source of replacement cells in regenerative medicine, but their plasticity and unlimited capacity for self-renewal raises concerns about their safety, including tumour formation ability, potential immune rejection, and the risk of differentiating into unwanted cell types. We report the medium-term to long-term safety of cells derived from human embryonic stem cells (hESC) transplanted into patients.
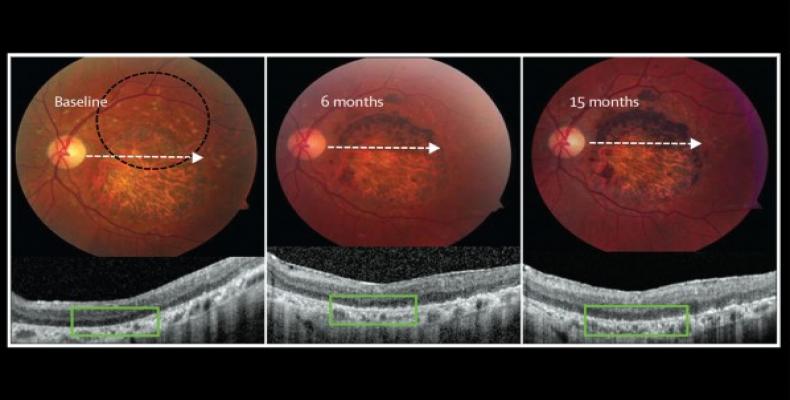
Two prospective phase 1/2 studies were done in the USA to assess the primary endpoints safety and tolerability of subretinal transplantation of hESC-derived retinal pigment epithelium in nine patients with Stargardt’s macular dystrophy (age over 18 years) and nine with atrophic age-related macular degeneration (age over 55 years). Three dose cohorts (50 000, 100 000, and 150 000 cells) were treated for each eye disorder. Transplanted patients were followed up for a median of 22 months by use of serial systemic, ophthalmic, and imaging examinations.
There was no evidence of adverse proliferation, rejection, or serious ocular or systemic safety issues related to the transplanted tissue. Adverse events were associated with vitreoretinal surgery and immuno-suppression. 13 (72%) of 18 patients had patches of increasing subretinal pigmentation consistent with transplanted retinal pigment epithelium. Best-corrected visual acuity, monitored as part of the safety protocol, improved in ten eyes, improved or remained the same in seven eyes, and decreased by more than ten letters in one eye, whereas the untreated fellow eyes did not show similar improvements in visual acuity. Vision-related quality-of-life measures increased for general and peripheral vision, and near and distance activities, improving by 16–25 points 3–12 months after transplantation in patients with atrophic age-related macular degeneration and 8–20 points in patients with Stargardt’s macular dystrophy.
The results of this study provide the first evidence of the medium-term to long-term safety, graft survival, and possible biological activity of pluripotent stem cell progeny in individuals with any disease. Our results suggest that hESC-derived cells could provide a potentially safe new source of cells for the treatment of various unmet medical disorders requiring tissue repair or replacement.
The research is funded by Advanced Cell Technology, Incorporated (ACT), a biotechnology company located in Marlborough, Massachusetts.