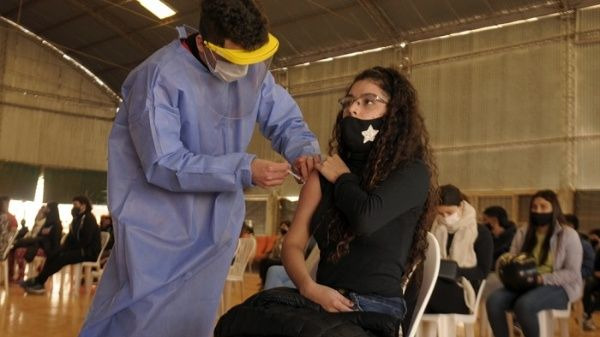
Argentina's Ministry of Health decided to expand its strategic plan for immunization against the SARS-CoV-2 virus.
Buenos Aires, July 24 (RHC)-- The Ministry of Health of Argentina reported on Friday that it will expand its immunization plan and will start vaccinating adolescents between 12 and 17 years old with the Moderna laboratory immunizer, prioritizing those who present risk factors.
In a statement published on its website, the Ministry announced that there is a consensus of the ministers of the whole country for its use in this age group, after the approval issued just this Friday by the European Medicines Agency (EMA).
The health authorities of Argentina added that the National Immunization Commission also recommends the use of the drug in this population group, which is why the decision was taken to extend the Strategic Vaccination Plan against COVID-19.
According to Ministry of Health estimates, there are 4.25 million adolescents in the country. Of these, 900,000 have comorbidities and will be prioritized, so 1.8 million doses will be required for the full immunization schedule.
Based on these calculations, part of the 3.5 million doses of Moderna donated by the U.S. last week will be used. It is now up to the ministers of health from all over the country to evaluate how the enrollment of young people between 12 and 17 years of age with comorbidities will be handled in order to face the vaccination process. This issue will be discussed at a meeting of the Federal Health Council scheduled for July 27.
Previously, representatives of the Moderna laboratory presented the results of their clinical trials in adolescents to the Argentine health authorities by means of a videoconference.
The Ministry of Health's press release points out that during that meeting, the members of the National Immunization Commission were inclined to recommend the "use of vaccines with mRNA platform in people from 12 to 17 years of age, prioritizing those who belong to risk groups".
The Moderna vaccine has messenger RNA (mRNA), a molecule which carries instructions to produce the Spike protein present in the SARS-CoV-2 coronavirus (the cause of Covid-19) to activate the defenses of the human organism when it enters with the virus.
According to the press release, this vaccine "has been shown to be a safe and highly effective product for this population group, in line with studies previously conducted in adults. The study was carried out in the United States with more than 3,000 participants between 12 and 17 years of age," it added.

